Working as a physician or doctor during these pandemic times is immensely more challenging than ever before, especially when providing the ultimate care to patients according to the evidence produced from clinical research and clear judgment. So, you should have access in creating worthwhile decisions to enable the translation of research into actions. But sadly, there still exists enormous gaps in different areas of medicine. In this article, we have some downloadable plan samples to guide you in your research work. Keep on reading!
FREE 10+ Clinical Research Project Plan Samples
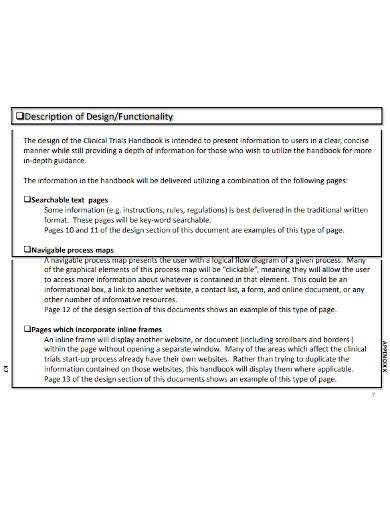
1. Clinical Research Project Plan Template
2. Clinical Research Pharmacy Project Plan
3. Sample Clinical Research Project Plan
4. Clinical Research Project Improvement Plan
5. Basic Clinical Research Project Plan
6. Clinical Research Project Development Plan
7. Clinical Research Project Planning
8. Simple Clinical Research Project Plan
9. Clinical StudyProject Plan Template
10. Managing Clinical Trials Sample
What is a Clinical Research Project Plan?
A clinical research project plan is a significant tool that helps fulfill the primary objectives and scope of the project for clinical research work. It is a document to present the mission and vision of a medical business firm, a pharmaceutical company, or a healthcare organization especially when there’s a need for in-depth clinical research when there occurs a sudden spread of viral diseases or to prevent the growth of other health issues in our society.
How to Write a Clinical Research Project Plan
Albert Szent-Györgyi, a Hungarian biochemist well-known for his contribution as the first person to isolate vitamin C and discover various components of citric acid, said: “Scientific inquiry is seeing what everyone else is seeing, but thinking of what no one else has thought.” Although, many people know what they know and acknowledge what they don’t know, we’re still having difficulties in not knowing the things we do not know. For example, we are still unaware of what’s the antidote for COVID-19 to quickly end today’s pandemic.
Thus, it is very important to empower you and your fellow doctors and physicians out there to deeply appreciate a research study’s strengths and weaknesses. Below are some helpful tips in writing an effective clinical research project plan:
1. Contemplate your experience in clinical research
Brainstorm all the medical practices you have done in your previous or current clinical work. Create a list of the possible clinical problems, the solutions you created, and your potential reaction to identical conditions after many years or a decade. Write a separate list of medical success stories that you can likely repeat in the future.
2. List your goals and outline your medical project
You need to create another list consisting of your main goals for your clinical research or medical project that you’re working on. Then, if you’re done with this, make a comprehensive outline of the scope of your overall research project.
3. Include a detailed timeline
Don’t forget to include a detailed timeline when you start working on your clinical research project. Also, describe how long will different procedures and methods of your clinical research be completed.
4. Determine the roles, responsibilities, and resources
In your clinical research, there are usually some medical professionals and other clinical research assistants that you may assign in various roles and responsibilities. So, determine the specific tasks that they need to take care of and record their names and responsibilities in your project plan. Plus, add the resources and references you and your research team have used for the project.
FAQs
According to National Comprehensive Cancer Network, the different stages of clinical trials are as follows:What are the different stages of clinical trials?
An ICF is the document with which the subjects and/or their representatives confirm that they have a legal agreement to participate in a clinical trial.What is ICF in clinical research?
Project management in the field of clinical research is used to properly manage all stages of a clinical trial, making sure that the objectives of the trial are reached on time, on budget, and based on the GCP.What is project management in clinical trials?
The five phases of project management are conceptualization and beginning, planning, implementation, performance or monitoring, and project conclusion.What are the 5 phases of a project?
Therefore, writing a project plan is a fundamental step in conducting and interpreting clinical research. As a doctor, you need to exemplify the imperative trait of being constantly productive in clinical care while cultivating excellence in the design and conduct of clinical research. To help you in this matter, you can freely use our guides with the tips we provided in this article. Here are some of our downloadable and printable project plan samples available in different kinds of formats. Simply click the templates in this article and start downloading now!
Related Posts
FREE 10+ Research Assistant CV Samples & Templates in MS Word
FREE 45 SOP Templates in PDF MS Word
FREE 4+ Coaching Skills Checklist Samples in PDF DOC
FREE 8+ Task List Templates in PDF MS Word
FREE 33+ Action Plan Samples in PDF
FREE 10+ Sample Risk Management Plan Templates in PDF
FREE 11+ Nursing Care Plan Templates in PDF MS Word
FREE 14+ Project Management Checklist Samples in PDF DOC
FREE 36+ Sample White Paper Templates in PDF
FREE 10+ Project Charter Samples in PDF DOC
FREE 9+ Healthcare Marketing Plan Samples in PDF MS Word
FREE 10+ Critical Review Samples in PDF MS Word
Sample Psychology Consent Form
FREE 8+ Evaluation Plan Templates in MS Word PDF
FREE 9+ Sample Need Assessment Templates in MS Word PDF